Plaintiffs Bring Motion to Centralize Port Catheter Litigation
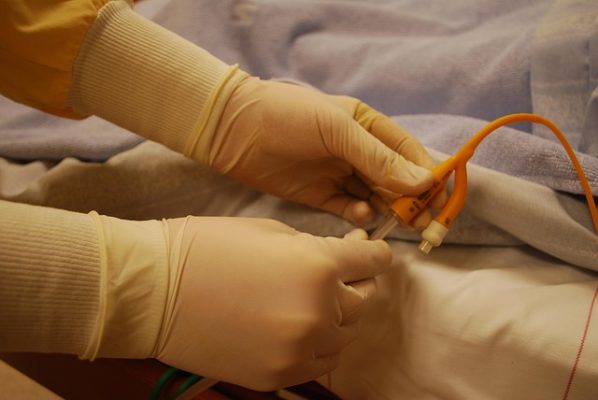 When there are a number of different cases pending in various districts against common defendants and featuring common factual and legal issues, it may be appropriate for plaintiffs to seek to have the cases centralized in a single district. When cases are centralized in this way, pretrial proceedings and discovery can be streamlined. Centralization also prevents judges’ inconsistent rulings on various pretrial matters, particularly evidentiary challenges. Recently, the plaintiffs in 19 lawsuits moved to centralize the port catheter litigation in the Western District of Missouri. These are lawsuits brought against defendants AngioDynamics, Inc., Navilyst Medical Inc., and PFM Medical, Inc in connection with the port catheters they manufacture—these are medical devices used to obtain easy access to veins and administer long-term intravenous treatments like chemotherapy. Patients have reported serious injuries believed to be the result of the devices. Many product liability lawsuits have been filed against the manufacturers.
When there are a number of different cases pending in various districts against common defendants and featuring common factual and legal issues, it may be appropriate for plaintiffs to seek to have the cases centralized in a single district. When cases are centralized in this way, pretrial proceedings and discovery can be streamlined. Centralization also prevents judges’ inconsistent rulings on various pretrial matters, particularly evidentiary challenges. Recently, the plaintiffs in 19 lawsuits moved to centralize the port catheter litigation in the Western District of Missouri. These are lawsuits brought against defendants AngioDynamics, Inc., Navilyst Medical Inc., and PFM Medical, Inc in connection with the port catheters they manufacture—these are medical devices used to obtain easy access to veins and administer long-term intravenous treatments like chemotherapy. Patients have reported serious injuries believed to be the result of the devices. Many product liability lawsuits have been filed against the manufacturers.
Call Moll Law Group About Your Port Catheter Products Claim
The port catheter litigation involves 23 pending lawsuits in 16 districts. There are 33 possible tag-along actions. Defendants AngioDynamics, Inc. and Navilyst Medical Inc. opposed centralization in Missouri. Alternatively, they suggested centralizing the actions in the Northern District of New York, the Southern District of California, or the Middle District of Alabama. Likewise, defendant PFM Medical, Inc., opposed the plaintiffs’ motion and suggested centralization in the Southern District of California and didn’t object to centralization in the Northern District of New York.
The United States Judicial Panel found that the lawsuits involved common factual questions and that centralizing the matters in the Southern District of California would serve the parties’ and witness’ convenience and promote just and efficient conduct in the lawsuits.
 Illinois Injury and Mass Tort Lawyer Blog
Illinois Injury and Mass Tort Lawyer Blog





 The United States Department of Agriculture’s Food Safety and Inspection Service (FSIS)
The United States Department of Agriculture’s Food Safety and Inspection Service (FSIS)  Recently, on May 29, 2025, the
Recently, on May 29, 2025, the  A
A  The FDA has warned consumers not to sell, serve or eat the distributor August Egg Company’s brown cage free and brown certified organic chicken eggs—1.7 million of them been
The FDA has warned consumers not to sell, serve or eat the distributor August Egg Company’s brown cage free and brown certified organic chicken eggs—1.7 million of them been  Seasonal allergies are triggered by pollen and blooming flowers and other plants. Allergy symptoms include runny noses, itchy eyes, sneezing, and sometimes coughing. Patients have been prescribed medications, and in some cases, take over-the-counter allergy medication.
Seasonal allergies are triggered by pollen and blooming flowers and other plants. Allergy symptoms include runny noses, itchy eyes, sneezing, and sometimes coughing. Patients have been prescribed medications, and in some cases, take over-the-counter allergy medication.  On May 1, 2025, SharkNinja Foodi OP Series Multi-Function Pressure Cookers were
On May 1, 2025, SharkNinja Foodi OP Series Multi-Function Pressure Cookers were  Two Alzheimer’s drugs Leqembi and Kisunla were approved by the FDA to treat mild dementia or a pre-Alzheimer’s condition called mild cognitive impairment. Data had suggested that Leqembi could slow cognitive decline in Alzheimer’s patients by five months over a period of 18 months for patients who had only mild symptoms. Two thousand volunteers had agreed to test Leqembi in clinical studies. However, it turns out these drugs may also increase the risk for brain bleeds and swelling in patients with certain genetic profiles—and patients may not have been given
Two Alzheimer’s drugs Leqembi and Kisunla were approved by the FDA to treat mild dementia or a pre-Alzheimer’s condition called mild cognitive impairment. Data had suggested that Leqembi could slow cognitive decline in Alzheimer’s patients by five months over a period of 18 months for patients who had only mild symptoms. Two thousand volunteers had agreed to test Leqembi in clinical studies. However, it turns out these drugs may also increase the risk for brain bleeds and swelling in patients with certain genetic profiles—and patients may not have been given  On February 20, 2025, Super 73
On February 20, 2025, Super 73  Recently, the Food and Drug Administration issued a
Recently, the Food and Drug Administration issued a