The Centers for Disease Control and Prevention Announce a Listeria Outbreak
 The Centers for Disease Control and Prevention recently announced that a listeria outbreak arising out of stone fruit—nectarines, peaches, and plums—made people in seven states sick. Up to 11 people reported they became sick due to the outbreak. Ten of those people who reported their sickness were hospitalized while one died. The United States Food and Drug Administration determined that there was a connection between the nectarines, plums, and peaches from HMC Farms that were distributed and sold in retail stores around the country between May – Nov. 15 in 2022 and 2023. If you suffered listeria and other ill effects from fruit, you should call the seasoned Chicago-based Moll Law Group about what happened. Billions have been recovered in lawsuits with which we’ve been involved.
The Centers for Disease Control and Prevention recently announced that a listeria outbreak arising out of stone fruit—nectarines, peaches, and plums—made people in seven states sick. Up to 11 people reported they became sick due to the outbreak. Ten of those people who reported their sickness were hospitalized while one died. The United States Food and Drug Administration determined that there was a connection between the nectarines, plums, and peaches from HMC Farms that were distributed and sold in retail stores around the country between May – Nov. 15 in 2022 and 2023. If you suffered listeria and other ill effects from fruit, you should call the seasoned Chicago-based Moll Law Group about what happened. Billions have been recovered in lawsuits with which we’ve been involved.
Consult the Seasoned Moll Law Group About Your Claim
HMC Farms, responding to the FDA announcement, issued a recall for certain conventionally grown fruit. Organic fruit was not recalled. And nectarines, plums and peaches sold at retail and grocery stores were not a part of the recall. The announcement from them noted that the fruit could be contaminated with Listeria monocytogenes, an organism that can trigger severe and even fatal infections, particularly in those who are pregnant or immune-compromised. When the affected person is pregnant, listeria can lead to losing the baby, giving birth prematurely, or giving the baby a life-threatening infection.
The announcement asked customers to take a look in their freezers to see if they had any of the fruit that was affected or had frozen it for later use.
 Illinois Injury and Mass Tort Lawyer Blog
Illinois Injury and Mass Tort Lawyer Blog





 The Supreme Court
The Supreme Court  Recently, manufacturer WanaBana agreed with the FDA to voluntarily
Recently, manufacturer WanaBana agreed with the FDA to voluntarily  The Joint Commission has
The Joint Commission has  Recently, Eli Lilly
Recently, Eli Lilly  Recently, a jury found the company Bayer liable in a product liability
Recently, a jury found the company Bayer liable in a product liability  Manufacturer Medline Industries initiated a
Manufacturer Medline Industries initiated a  Manufacturer Kraft Heinz discovered that one of the machines that individually wraps Kraft Singles American processed cheese slices permits thin strips of film to say on the slices even after removal of the wrapper. The food company announced a
Manufacturer Kraft Heinz discovered that one of the machines that individually wraps Kraft Singles American processed cheese slices permits thin strips of film to say on the slices even after removal of the wrapper. The food company announced a  The Food and Drug Administration recently updated the
The Food and Drug Administration recently updated the 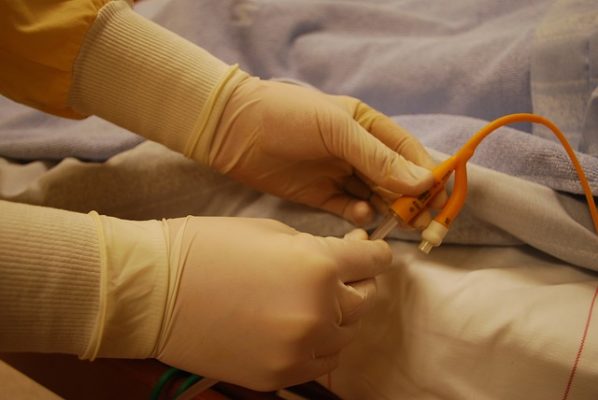 Bard’s PowerPort devices are implanted under the skin and pump intravenous chemotherapy and other fluid into a patient’s bloodstream. These are two-part devices with an injection reservoir and a catheter. Recently, plaintiffs in eight lawsuits against Becton, Dickinson & Company, C.R. Bard, Inc., and Bard Access Systems
Bard’s PowerPort devices are implanted under the skin and pump intravenous chemotherapy and other fluid into a patient’s bloodstream. These are two-part devices with an injection reservoir and a catheter. Recently, plaintiffs in eight lawsuits against Becton, Dickinson & Company, C.R. Bard, Inc., and Bard Access Systems