AngioDynamics, Inc., Navilyst Medical Inc. and PFM Medical, Inc. Port Catheter
Chicago-Based Lawyers for Defective Port Catheter Claims
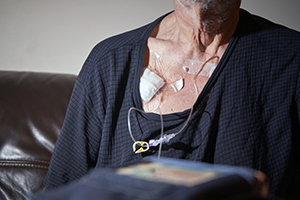 Port catheters made by AngioDynamics, Inc., Navilyst Medical Inc., and PFM Medical Inc. are devices that are used by medical providers to administer long term intravenous treatments such as chemotherapy. These devices allow for easier access to veins. Their benefits may be outweighed, however, by their potential to cause injuries. Patients have reported suffering a range of injuries from blood clots to infections and thrombosis as a result of flaws in the product. If you were injured by an AngioDynamics port catheter or another manufacturer’s port catheter, you should call the trustworthy Chicago-based lawyers of Moll Law Group. Billions have been recovered in cases with which we’ve been involved. We represent clients around the nation.
Port catheters made by AngioDynamics, Inc., Navilyst Medical Inc., and PFM Medical Inc. are devices that are used by medical providers to administer long term intravenous treatments such as chemotherapy. These devices allow for easier access to veins. Their benefits may be outweighed, however, by their potential to cause injuries. Patients have reported suffering a range of injuries from blood clots to infections and thrombosis as a result of flaws in the product. If you were injured by an AngioDynamics port catheter or another manufacturer’s port catheter, you should call the trustworthy Chicago-based lawyers of Moll Law Group. Billions have been recovered in cases with which we’ve been involved. We represent clients around the nation.
Call Moll Law Group About Your Claim Against AngioDynamics, Inc., Navilyst Medical Inc., or PFM Medical, Inc.
Reports of defects that cause serious injury have led to litigation against the manufacturers for failure to warn of risks, among other claims. AngioDynamics is the manufacturer of SmartPort, Vortx, Xcela, Vaxcel and other port catheters. Navilyst is its subsidiary. Injuries that plaintiffs have reported have included fractures to the port catheter that result in pieces moving to other places in the body, including the heart, and causing dangerous complications and the need for emergency surgery and infections even after there has been proper installation of SmartPort.
The FDA has taken many reports of injuries, including infections, blood clots, and perforations, that it records in its MAUDE (Manufacturer and User Facility Device Experience) database. In addition, there are at least 23 pending lawsuits in 15 districts and 33 possible tag-along actions that were centralized in multidistrict litigation in the Southern District of California by the United States Judicial Panel based on their common factual and legal questions.
Plaintiffs allege that the defendants manufacture the catheter portion of their port devices with too high a concentration barium sulfate and that as a result, the catheters are susceptible to cracking and pitting and degraded materials, which leads, in turn, to fractures. Additionally, the AngioDynamic port catheter and other port catheter cases in the multidistrict litigation involve shared factual questions with regard to manufacturing, design marketing, development, and sale of the devices in question.
Recovering Damages Against Manufacturers
Sometimes the injuries sustained as a result of port catheter devices such as the ones made by AngioDynamics, Navilyst and PFM are quite serious and require expensive medical care, including revision surgeries. If you were injuries by one of these port catheters, you may be able to obtain relief by pursuing damages in a product liability lawsuit. This kind of lawsuit can be mounted if a product has defects in its manufacturing, design, or marketing. Generally speaking, manufacturing defect occur only in particular batches of a product because they are flaws in how the product was made. Meanwhile, design defects are flaws that are in every unit of the product—as the name suggests, they are flaws in specifications for making the products. For instance, if the instructions for making the product require too high a concentration of barium sulfate and this is what causes cracking of the device, there may be a design flaw. In many instances, marketing defects involve inadequate, or no warnings of risks attached to a product.
If our attorneys can establish AngioDynamics, Navilyst Medical Inc. or PFM’s liability for a defective port catheter, we may be able to recover compensatory damages on your behalf. These damages may be both economic and noneconomic. They can include medical bills, lost wages, lost earning capacity, pain and suffering, emotional distress, loss of enjoyment of life, and replacement services.
Contact Our Trusted Chicago-based AngioDdynamics Port Catheter Attorneys
If you or a loved one suffered injuries that were caused by port catheters made by AngioDynamics, Navilyst Medical Inc. or PFM Medical, Inc., call the knowledgeable Chicago-based product liability lawyers of Moll Law Group about whether you have a viable basis to pursue damages in a lawsuit. We are dedicated to fighting for injured consumers and patients around the United States. Complete our online form or call us at 312.462.1700.
 Moll Law Group Home
Moll Law Group Home