Senate Passes Long Anticipated PACT Act
 On August 2, 2022, the Senate passed the long-sought PACT Act. The passage of such bill marks the end of a persistent fight to get the law passed through Congress. This legislation had been held up in the chamber after opposition from more than a dozen Republicans. After extensive backlash from veterans and veterans’ groups, the final vote was 86-11 and sent to President Joe Biden to sign into law.
On August 2, 2022, the Senate passed the long-sought PACT Act. The passage of such bill marks the end of a persistent fight to get the law passed through Congress. This legislation had been held up in the chamber after opposition from more than a dozen Republicans. After extensive backlash from veterans and veterans’ groups, the final vote was 86-11 and sent to President Joe Biden to sign into law.
Talk to Moll Law Group About Whether You Have a Claim
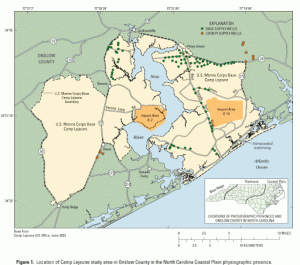
The PACT Act will expand health care benefits for millions of veterans exposed to toxic substances during their military service. The bill will also include the highly anticipated Camp Lejeune Justice Act of 2022. The Camp Lejeune Justice Act addresses the water contamination at Marine Corps Base Camp Lejeune in North Carolina between August 1, 1953 and December 31, 1987. It is important to note that this action will only be available for individuals exposed to the contaminated water for at least thirty (30) days. Individuals who have sustained injuries due to their exposure will finally be able to file suit for monetary compensation.
 Illinois Injury and Mass Tort Lawyer Blog
Illinois Injury and Mass Tort Lawyer Blog





 Recently, RIO-branded swinging hammock chairs were
Recently, RIO-branded swinging hammock chairs were  Camp Lejeune is a military training facility for the United States Marine Corps. Water treatment facilities that supplied water to Camp Lejeune Marine Corps base were contaminated. The volatile organic compounds dumped there from 1957 – 1987 may have caused serious injuries to Marines, Sailors, and their families on the base. Civilian employees may have been affected, too. Recently, a
Camp Lejeune is a military training facility for the United States Marine Corps. Water treatment facilities that supplied water to Camp Lejeune Marine Corps base were contaminated. The volatile organic compounds dumped there from 1957 – 1987 may have caused serious injuries to Marines, Sailors, and their families on the base. Civilian employees may have been affected, too. Recently, a  Sometimes baby neck floats are used on babies with spina bifida, spinal muscular atrophy (SMA) type 1, cerebral palsy, and Down syndrome. The United States Food and Drug Administration (FDA) recently issued
Sometimes baby neck floats are used on babies with spina bifida, spinal muscular atrophy (SMA) type 1, cerebral palsy, and Down syndrome. The United States Food and Drug Administration (FDA) recently issued  Thirteen models of Frigidaire refrigerators and an Electrolux model refrigerator have been
Thirteen models of Frigidaire refrigerators and an Electrolux model refrigerator have been  Recently, Medtronic
Recently, Medtronic  On June 23, 2022, The U.S. Food and Drug Administration
On June 23, 2022, The U.S. Food and Drug Administration  A broad range of chronic conditions are treated with the Volara System, which provides continuous positive expiratory pressure (CPEP) to expand your lungs and airways, along with continuous high-frequency oscillation pulses to free mucus plugs and make it easier to cough and breathe less effortfully. However, if you use the Volara System, it’s important to be aware that its manufacturer, Baxter International Inc., issued an Urgent Medical Device Correction for the Volara System and then a
A broad range of chronic conditions are treated with the Volara System, which provides continuous positive expiratory pressure (CPEP) to expand your lungs and airways, along with continuous high-frequency oscillation pulses to free mucus plugs and make it easier to cough and breathe less effortfully. However, if you use the Volara System, it’s important to be aware that its manufacturer, Baxter International Inc., issued an Urgent Medical Device Correction for the Volara System and then a 
 Parents may be alarmed to learn that Asweets has recalled its wooden baby activity push walker due to a strangulation hazard. The rubber rings on the rear wheels of a walker may separate from the wheels and detach, and this can result in a young child being at risk of strangulation. Asweets has received 10 reports of the rubber rings detaching from the wheels, though no injuries to babies have been reported. On June 2, 2022, the company
Parents may be alarmed to learn that Asweets has recalled its wooden baby activity push walker due to a strangulation hazard. The rubber rings on the rear wheels of a walker may separate from the wheels and detach, and this can result in a young child being at risk of strangulation. Asweets has received 10 reports of the rubber rings detaching from the wheels, though no injuries to babies have been reported. On June 2, 2022, the company